Buy Gabapentin Online – COD
Gabapentin works in the brain to prevent seizures and relieve pain for certain conditions in the nervous system. It is not used for routine pain caused by minor injuries or arthritis. Gabapentin is an anticonvulsant. This medicine is available only with your doctor’s prescription.
| Product Name | Total | Order |
| Gabapentin 800 mg – 180 Tabs | $189 | Order |
| Gabapentin 600 mg – 180 Tabs | $186 | Order |
| Gabapentin 400 mg – 180 Tabs | $179 | Order |
| Gabapentin 300 mg – 180 Tabs | $169 | Order |
| Generic Fioricet – 90 Tabs | $169 | Order |
| Generic Fioricet – 120 Tabs | $199 | Order |
| Generic Fioricet – 180 Tabs | $229 | Order |
We guarantee the best Gabapentin, and generic fioricet, butalbital apap caffeine online at the cheapest prices. We provide free delivery using USPS priority mail delivery service by COD payment services. You can know how to order by credit card if you have successfully ordered in our COD US licensed pharmacy.
Our online pharmacy offers you to buy original Neurontin and its high quality generics at much lower prices. Generics of Neurontin are significantly cheaper in comparison with the brand medicine.
We do have credit card processing for Gabapentin and fioricet delivery. But it is only for COD returned customers. When you have successfully picked up more than two COD packages, we will tell you our credit card processing website address.
We have several warehouses and some of them list GAB as not shipped DNS in some states. But we can ship to your states by refer you to different warehouses.
Welcome to our US licensed online pharmacy – buyinggabapentin.com – bringing quality, affordable healthcare products from our pharmacies to your door. Order your prescription drugs from buyinggabapentin.com and benefit from:
1. Discreet, no cost medical consultations with US licensed doctors and pharmacists
2. 100% FDA approved generic and branded prescription drugs sourced in the U.S.
3. Free USPS Priority Mail shipping
4. The security and accountability of a U.S. owned and operated business
5. Your Privacy
6. Free Prescription by US licensed doctors
7. You can order at any time and do not need drive to your local pharmacies.
8. Save you Money
9. SSL Technology
10. We do not store customer information online
11. We never refill orders without your approval
12. We do card processing for returned customers
13. Save your driving time
Buyinggabapentin.com does not dispense nor prescribe medication directly. It is still US licensed pharmacies who has the final authorization to approve or deny prescription requests. We can submit your orders manually into our upstream warehouse portal, the warehouse has several pharmacies to work with. The warehouse will dispatch your orders to different pharmacies. Normally, The pharmacies can ship your orders the same day after the physicians review your order. But sometimes, the order will be shipped the following business day.
We are especially good at Gabapentin Delivery. You will not find other authentic gabapentin pharmacies have so cheapest prices.
Gabapentin is available in over 40 countries. It is a prescription drug, marketed as Neurontin and Horizant, that’s used to treat epilepsy. Doctors can prescribe gabapentin to treat epilepsy in people older than 12, and partial seizures in children ages 3 to 12.
Gabapentin may also be prescribed to treat restless legs syndrome (RLS), to relieve numberness and tingling related todiabetes, to prevent hot flashes, and to relieve pain that can accompany shingles (known as postherpetic neuralgia).
Studies have also found Gabapentin have a substantial analgesic effects on diabetic neuropathy, postherpetic neuralgia, migraine, and other neuropathic pain conditions, as well as beneficial effect on sleep and restless legs syndrome. On the basis of these findings, some doctors also prescribe gabapentin to cure fibromyalgia pain. For more information, please click here.
Gabapentin is an effective prophylactic agent for patients with migraine. In addition, gabapentin appears generally well tolerated with mild to moderate somnolence and dizziness.
Pregabalin (Lyrica), a drug similar to gabapentin, was the first medication approved by the Food and Drug Administration (FDA) to treat fibromyalgia. While gabapentin hasn’t been approved by the FDA for the treatment of fibromyalgia, some doctors may prescribe it off-label for such use.
Anticonvulsant drugs, such as gabapentin, are becoming increasingly popular for migraine prevention.
Gabapentin has mush more usages, it can cure nerve pain, prevent migraines and headaches. It is also widely used to treat Insomnia, Fibromyalgia, and Restless Legs Syndrome Pain. For more information, please check What is Gabapentin and What It Is Used For ?
A study in the Canadian Journal of Anesthesia in 2013 revealed that gabapentin may help ease moderate to high levels of anxiety among people about to have surgery. The researchers noted that doctors are increasingly using the drug to treat pain after surgery as well as a variety of psychiatric diseases, such as chronic anxiety disorders.
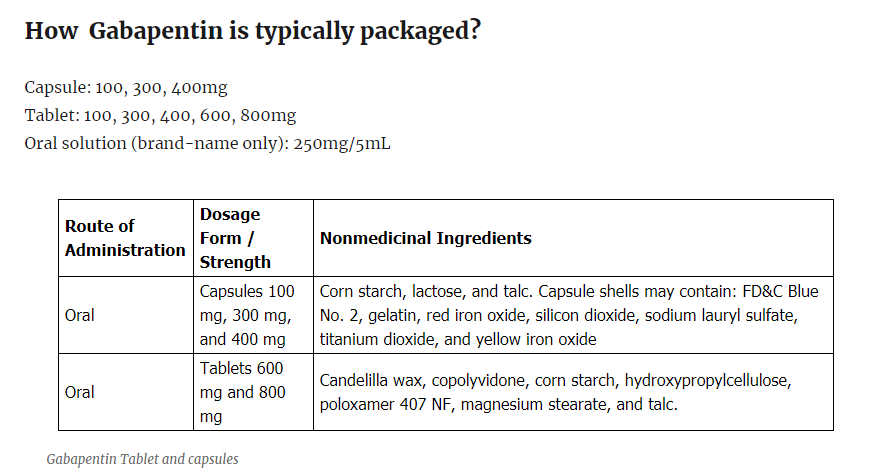
How Gabapentin is typically packaged?
Capsule: 100, 300, 400mg
Tablet: 100, 300, 400, 600, 800mg
Oral solution (brand-name only): 250mg/5mL

Neurontin is a trade mark that is owned by Pfizer company. Most of the Neurontin we provided is generic Neurontin. It is also called Gabapentin. Gabapentin has absolutely the same compound, effect and safety level as Neurontin. Gabapentin is much cheaper than Neurontin.
Our pharmacies are US licensed pharmacies and our doctors are US licensed doctors. We can guarantee the Neurontin at our online pharmacy has the highest quality as well as affordable price at the same time.
What you must know before you order Gabapentin Online
You should know that gabapentin may increase the risk for suicide. Suicidal thoughts or behavior occurs in about one in 500 people taking medications like gabapentin.
FDA warns about serious breathing problems with seizure and nerve pain medicines gabapentin (Neurontin, Gralise, Horizant) and pregabalin (Lyrica, Lyrica CR)
This risk may begin within a week of starting treatment.
Let your doctor know if you experience:
- Thoughts of suicide
- Symptoms of depression
- Aggression
- Irritability
- Panic attacks
- Extreme worry
- Restlessness
- Acting without thinking
- Abnormal excitement
- Alcohol or other drug addictive
You should also let friends and family members know about these symptoms. We do not suggest you buy Gabapentin online without your local doctor’s prescription. You must have knew the side effects of Gabapentin before you buy Gabapentin online.
You should also know we only send you orders when you have your local doctor prescribed you first prescription and we can only refill Gabapentin for you.
The FDA placed Gabapentin in pregnancy category C. According to studies done on animals, there was harm done to fetuses. However, there have been no studies done on humans. Despite all this, experts believe that the benefits gained from taking Gabapentin may outweigh its risks.
If you want to stop taking gabapentin, please stop it gradually. Like other psychotropic drugs, people should ease off Gabapentin gradually. There are some known withdrawal symptoms. This mostly comes from people who take high doses of the drug and suddenly stop. People should only abruptly discontinue Gabapentin because of a serious side effect.
Gabapentin Mechanism Of Action
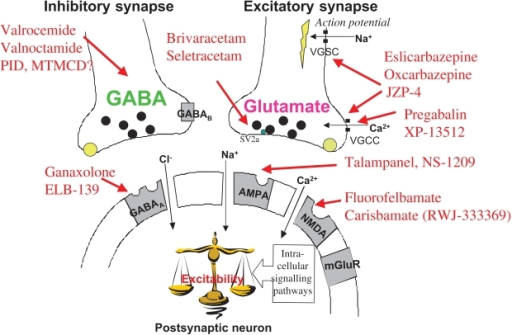
The precise mechanisms by which gabapentin produces its analgesic and antiepileptic actions are unknown. Gabapentin is structurally related to theneurotransmitter gamma-aminobutyric acid (GABA) but has no effect on GABA binding, uptake, or degradation. In vitro studies have shown that gabapentin binds with high-affinity to the α2δ subunit of voltage-activated calcium channels; however, the relationship of this binding to the therapeutic effects of gabapentin is unknown.
Do not stop taking NEURONTIN without first talking to your healthcare provider. Stopping NEURONTIN suddenly can cause serious problems.
Gabapentin Side effects
NEURONTIN can cause serious side effects including:
1. Suicidal Thoughts. Like other antiepileptic drugs, NEURONTIN may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempts to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop taking NEURONTIN without first talking to a healthcare provider.
- Stopping NEURONTIN suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
- Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
2. Changes in behavior and thinking –Using NEURONTIN in children 3 to 12 years of age can cause emotional changes, aggressive behavior, problems with concentration, restlessness, changes in school performance, and hyperactivity.
3. NEURONTIN may cause serious or life-threatening allergic reactions that may affect your skin or other parts of your body such as your liver or blood cells. This may cause you to be hospitalized or to stop NEURONTIN. You may or may not have a rash with an allergic reaction caused by NEURONTIN. Call a healthcare provider right away if you have any of the following symptoms:
- skin rash
- hives
- difficulty breathing
- fever
- swollen glands that do not go away
- swelling of your face, lips, throat, or tongue
- yellowing of your skin or of the whites of the eyes
- unusual bruising or bleeding
- severe fatigue or weakness
- unexpected muscle pain
- frequent infections
These symptoms may be the first signs of a serious reaction. A healthcare provider should examine you to decide if you should continue taking NEURONTIN.
The most common side effects of gabapentin in adult patients include dizziness, fatigue, drowsiness, weight gain, and peripheral edema (swelling of extremities).[40] Gabapentin may also produce sexual dysfunctionin some patients, symptoms of which may include loss of libido, inability to reach orgasm, and erectile dysfunction. Gabapentin should be used carefully in patients with renal impairment due to possible accumulation and toxicity. What side effects can Gabapentin cause?
Gabapentin Dosage
A typical adult dose for postherpetic neuralgia usually starts at 300 milligrams (mg), and your doctor may increase the dose to up to 1,800 mg a day.
A typical adult dose for epilepsy may range from 900 to 1,800 mg a day.
There are several online Gabapentin Dosage Available.
Gabapentin 800mg 180 tab
Gabapentin 600mg 180 tab
Gabapentin 400mg 180 tab
Gabapentin 300mg 180 tab
Normally Gabapentin 800mg is hard to get from online pharmacy because you have to gradually take gabapentin to solve your berve pain problems.
Usual Adult Dose for Postherpetic Neuralgia:
Initial dose: 300 mg orally on day one, 300 mg orally twice a day on day two, then 300 mg orally 3 times a day on day three.
The dose may be titrated up as needed for pain relief to a daily dose of 1800 mg.
Maintenance dose: 900 to 1800 mg orally in 3 divided doses.
Efficacy was demonstrated in clinical studies over a range of 1800 mg/day to 3600 mg/day. However, no additional benefit was demonstrated from the use of doses over 1800 mg/day.
Gabapentin available under the trade name Gralise (R):
Maintenance dose: Gralise (R) should be titrated to 1800 mg orally once daily with the evening meal.
Recommended titration schedule:
Day 1: 300 mg orally with the evening meal
Day 2: 600 mg orally with the evening meal
Days 3 through 6: 900 mg orally with the evening meal
Days 7 through 10: 1200 mg orally with the evening meal
Days 11 through 14: 1500 mg orally with the evening meal
Day 15: 1800 mg orally with the evening meal
Gralise (R) is not interchangeable with other gabapentin products because of differing pharmacokinetic profiles that affect the frequency of administration.
Gabapentin enacarbil extended release tablets available under the trade name Horizant (R):
The recommended dosage is 600 mg orally twice daily. Therapy should be initiated at a dose of 600 mg orally in the morning for 3 days of therapy, then increased to 600 mg twice daily (1,200 mg/day) on day four.
Gabapentin enacarbil extended release tablets available under the trade name Horizant (R) and gabapentin are not interchangeable.
Usual Adult Dose for Restless Legs Syndrome:
Gabapentin enacarbil available under the trade name Horizant (R):
600 mg orally once daily with food at about 5 PM
Usual Pediatric Dose for Epilepsy:
Less than 3 years: Effectiveness has not been established.
Greater than or equal to 3 and less than 12 years:
Starting Dose: ranges from 10 to 15 mg/kg/day in 3 divided doses.
Effective Dose: reached by upward titration over a period of approximately 3 days. The effective dose of gabapentin in patients 5 years of age and older is 25 to 35 mg/kg/day and given in divided doses (three times a day). The effective dose in pediatric patients ages 3 and 4 years is 40 mg/kg/day and given in divided doses (three times a day). Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been well tolerated in a long term clinical study. The maximum time interval between doses should not exceed 12 hours.
Greater than 12 years:
Initial dose: 300 mg orally on day one, 300 mg orally twice a day on day two, then 300 mg orally 3 times a day on day three.
Maintenance dose: 900 to 1800 mg orally in 3 divided doses. If necessary, the dose may be increased using 300 mg or 400 mg capsules three times a day up to 1800 mg/day. Dosages up to 2400 mg/day have been well tolerated in long term clinical studies. Doses of 3600 mg/day have also been administered to a small number of patients for a relatively short duration, and have been well tolerated. The maximum time between doses in the three times a day schedule should not exceed 12 hours.
Your doctor will usually start you at a low dose of gabapentin and then increase the dose gradually until you get to a level that works best for you. For More information, please check How should Gabapentin be used?
Gabapentin Overdose
A lethal dose of gabapentin was not identified in mice and rats receiving single oral doses as high as 8000 mg/kg. Signs of acute toxicity in animals included ataxia, labored breathing, ptosis, sedation, hypoactivity, or excitation.
Acute oral overdoses of NEURONTIN up to 49 grams have been reported. In these cases, double vision, slurred speech, drowsiness, lethargy, and diarrhea were observed. All patients recovered with supportive care. Coma, resolving with dialysis, has been reported in patients with chronic renal failure who were treated with NEURONTIN.
Gabapentin can be removed by hemodialysis. Although hemodialysis has not been performed in the few overdose cases reported, it may be indicated by the patient’s clinical state or in patients with significant renal impairment.
Gabapentin Abuse
Gabapentin abuse tends to occur in people who already have an addiction to opioids or other drugs. The effects of Gabapentin intoxication have been described as a sense of calm, euphoria, and a high similar to marijuana.
A 2013 study in Kentucky found that of the 503 participants reporting illegal drug use, 15% reported using Gabapentin in addition to other drugs to get high in the previous six months. Another study, working with a sample of participants meant to represent the national population, found almost a quarter of patients with co-prescriptions of opioids and Gabapentin were getting more than three times their prescribed amount to supply their addiction. People using the drug without a prescription is a growing problem in many areas. Due to the drug’s legal status, this is difficult to address from a policing standpoint. States where Gabapentin abuse is becoming more common are beginning to classify the drug as a more strictly controlled substance.
Gabapentin’s unique ability to address multiple ailments has made it one of the most popular prescription medications in the U.S. In May of 2019, GoodRx reported that it was the fifth-most prescribed drug in the nation. Despite its low abuse potential, its ability to be used in conjunction with other drugs causes widespread harm and addiction.
Online Gabapentin Brand Names
In the U.S.
- FusePaq Fanatrex
- Gabarone
- Gralise
- Neurontin
Available Dosage Forms:
- Tablet
- Capsule
- Suspension
- Solution
Therapeutic Class: Anticonvulsant
Chemical Class: Gamma Aminobutyric Acid (class)
The off-lable use of Gabapentin for migraine
Neurontin is prescribed for the treatment of the following conditions in adults and children over 3 years:
Various forms of epilepsy. When used for controlling epilepsy, it is usually used in conjunction with another anti-epileptic drug. Usually doctors prescribe prescribe Neurontin for patients to help them to treat your epilepsy when a current treatment is not fully controlling his/her condition. Neurontin is used as addition to the main treatment of epilepsy.
Peripheral neuropathic pain (long lasting pain caused by damaged nerves). This disease can occur and develop in various conditions: injury, diabetes, shingles, and others.
Your doctor may prescribe you Neurontin for the treatment of other diseases, if he thinks that it is a right medicine for your case which is offl-label use of Neurontin.
It is also widely used to treat Anxiety and Migraine prevention.
Gabapentin Off-Label Usage
One of Gabapentin “off-label” usage is for migraine prevention and treatment, including migraines with or without aura, vestibular migraines. It can reduce the frequency of headaches, pain intensity, and the use of symptomatic medications. Gabapentin is a good preventive therapy for migraines refractory to standard medications.
The chemical structure of gabapentin is related that of gamma-aminobutyric acid (GABA) which is a neurotransmitter in the brain. The exact mechanism as to how gabapentin controls epilepsy and relieves pain is unknown, but it probably acts like the neurotransmitter GABA.
The effective dose of gabapentin varies greatly. Some persons need only 200-300 mg a day whereas others may need 3000 mg or more a day. It may take several weeks to become effective, so it is important to stay on it for an adequate length of time.
The Efficacy of gabapentin in migraine prophylaxis experiment shows gabapentin is an effective prophylactic agent for patients with migraine.
In the Clinical trials, 143 patients evaluated gabapentin for migraine prophylaxis. After 3 months the patients taking gabapentin had a reduction of the migraine frequency by 1.5 migraines per month (or by 35.7%) compared with a reduction of 0.6 migraines per month for the placebo group. Also, gabapentin reduced the headache frequency by 50% or greater in 45% patients compared with only 16% patients on placebo. The most frequently reported adverse events were asthenia, dizziness, somnolence, and infection.
- Gabapentin Forms
- Gabapentin On Empty Stomach- Is It Really Safe?
- Gabapentin for Back Pain
- Gabapentin For Migraines – Is It Useful?
- How Long Does Gabapentin Stay In Your System?
- Is Gabapentin A Controlled Substance?
- How to Buy Gabapentin from Certified Pharmacy?
- Gabapentin Mechanism of Action
- Can I drive or ride a bike after I take Gabapentin ?
- Do I need to stay on the same brand of Gabapentin?
- How and when to take Gabapentin ?
- Risks of taking Gabapentin during pregnancy and when breastfeeding
- Presence of other health conditions that affect Gabapentin
- Gabapentin Interactions with other medications and substances
- What is the maximum daily dosage of Gabapentin?
- Does Gabapentin cause constipation?
- Does gabapentin help you sleep?
- Is gabapentin a narcotic/controlled substance?
- What happens when you suddenly stop taking gabapentin?
- Does Gabapentin Cause Weight Gain?
- How long does it take gabapentin to work?
- Is gabapentin considered a painkiller?
- Does gabapentin help nerve pain?
- Why Is Lyrica a Controlled Substance?
- What’s the difference between Lyrica and Gabapentin ?
- Gabapentin interactions with medicines
- NT16 Neurontin 600mg, NT26 Neurontin 800mg – Pfizer U.S. Pharmaceuticals Group
- T1 Gabapentin 600mg, T3 Gabapentin 800mg – Ascent Pharmaceuticals, Inc.
- NT16 Gabapentin 600mg, NT26 Gabapentin 800mg – Greenstone LLC
- G6 Gabapentin 600mg, G8 Gabapentin 800mg – Ascend Laboratories, LLC
- Logo 4443 600 (Gabapentin 600mg), Logo 4444 800 (Gabapentin 800 mg) – Teva Pharmaceuticals USA
- 2 04 Gabapentin 800mg, 2 02 Gabapentin 600mg – Sun Pharmaceutical Industries Inc.
- X 93 Gabapentin 800 mg, X 92 Gabapentin 600 mg – Ranbaxy Pharmaceuticals Inc.
- APO Gabapentin 600mg, APO Gabapentin 800mg – Apotex Corporation
- Gabapentin 600mg, 800mg – Greenstone Limited
- Gabapentin 800mg, Gabapentin 600mg – Actavis Pharma, Inc.
- Gabapentin 600mg, Gabapentin 800mg – InvaGen Pharmaceuticals, Inc
- Gabapentin 600mg, 800mg – Zydus Pharmaceuticals (USA) Inc.
- Gabapentin 600 mg, 800mg – Solco Healthcare U.S., LLC
- Gabapentin 600 mg, Gabapentin 800 mg – ScieGen Pharmaceuticals, Inc.
- Gabapentin 800mg , Gabapentin 600mg – Aurobindo Pharma USA, Inc.
- Gabapentin 600mg, 800mg – Glenmark Pharmaceuticals Inc.
- Gabapentin 800mg, Gabapentin 600mg – CSPC OUYI Pharmaceutical Co.
- New Migraine Treatment Methods
- Gabapentin Can be Used for Migraine Prevention
- Gabapentin ( Neurontin ) is Used Much More Extensively in the Medical Fields to Treat Nerve Pain Than it is to Treat Epilepsy
- What is the Signs of a Gabapentin Addiction and How to Treat Gabapentin Addiction
- Gabapentin reviews for restless legs syndrome
- Reviews of Gabapentin for Postherpetic Neuralgia
- Gabapentin reviews for treating Insomnia
- Gabapentin reviews for anxiety
- Gabapentin reviews for migraine prevention
- Reviews for Gabapentin to treat Bipolar Disorder
- Reviews for Gabapentin to treat Trigeminal Neuralgia
- Reviews for Gabapentin to treat Hot Flashes
- Reviews for Gabapentin to treat Occipital Neuralgia
- Gabapentin for Alcohol Withdrawal Reviews
- Gabapentin for Postmenopausal Symptoms Reviews
- Why is fioricet exempt ? Exempt Prescription Products List (2)
- Why is fioricet exempt ? Exempt Prescription Products List (1)
- Who can not buy gabapentin online ?
- Gabapentin 800mg Pictures and Gabapentin 800mg Manufacturers
- Gabapentin can be used for restless legs syndrome
- Gabapentin OVERDOSE and Gabapentin Abuse
- What is the Gabapentin Interactions
- Gabapentin Improves Menopausal Hot Flashes, Insomnia
- Treatment effects of gabapentin for primary insomnia
- Gabapentin FDA approval
- What side effects can Gabapentin cause?
- What special precautions should I follow before I take Gabapentin?
- How should Gabapentin be used?
- What are the ingredients in NEURONTIN?
- Neurontin can be used to treat Fibromyalgia, Migraine, sleep
- Gabapentin is the best medications to treat Fibromyalgia
- What is Gabapentin and What It Is Used For ?
- Efficacy of gabapentin in Preventing Migraine
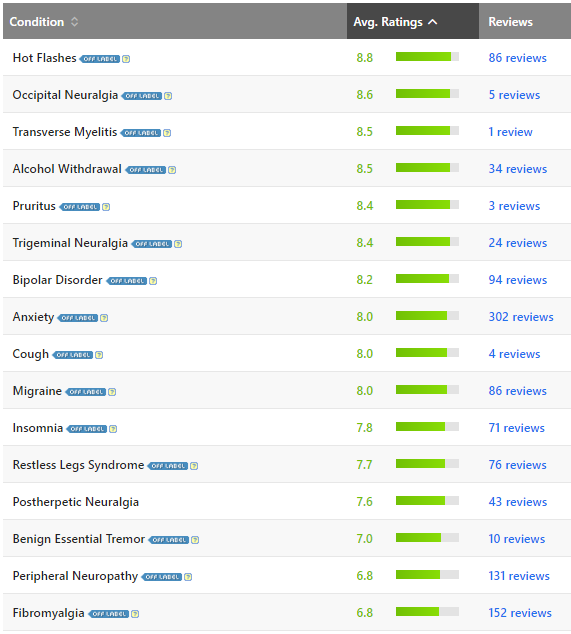
Gabapentin for Migrain patient Reviews
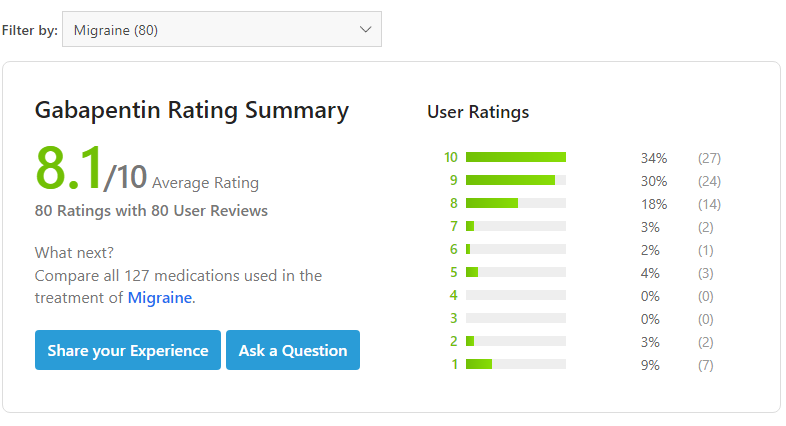
In Famous medical websites, migraine patients also review the gabapentin as the migraine prevention medicine. They rate Gabapentin 8.1 stars out of ten stars. It is a high mark and means Gabapentin is a very effective medicine for migraine prevention.
I haven’t been taking this medication for long but it’s helped so much. Neuro started me off on 300mg at night and now I’m at 600mg at night. It doesn’t make me sleepy or drowsy. Before starting gabapentin, I was having migraines just about every day. I started having aphasia and vision changes with my migraines, so I decided to take action. I’ve only been on it for almost 2 weeks but I’ve been migraine free and my triggers are no longer triggers at this point, which is fantastic. I should note it has reduced my appetite but this is not a negative thing.” – Crystaldreams July 25, 2017
Abuse of gabapentin is not widespread. Research literature indicates a few scattered accounts of abuse of the drug, mostly by individuals with psychiatric issues or pre-existing substance use disorder problems who are attempting to use the drug to enhance the effectiveness of other drugs or who cannot get their normal drug of abuse. Nonetheless, any medication can be abused by individuals, and someone using large amounts of gabapentin with or without a prescription should be suspected of abusing the drug.
Gabapentin and alcohol
Avoid taking Gabapentin with alcohol, because this drug is known to be an effective remedy used during alcoholism treatment. Simultaneous intake of the drug with alcohol may cause negative side effects.
Gabapentin withdrawal
Do not rapidly stop taking Gabapentin if you take it for a long time. The sudden termination of the drug intake can provoke withdrawal symptoms. The gradual dosage decline within weeks or months helps to minimize or prevent withdrawal symptoms. The side effects observed after the termination of Gabapentin intake include sleeplessness, anxiety, excitement, disorientation, confusion of consciousness, photosensitivity, sweating, headache, heartbeat, arterial hypertension.
Is Gabapentin used to get high?
The drug is not an opioid narcotic substance, that is why it is very unlikely you may experience such side effects as are observed after opioid drugs intake. However, the reaction depends on a definite person. Most common side effects of this kind are very mild. It may be, for instance, low blood pressure or dizziness.
Is Gabapentin addictive?
Gabapentin is not on a list of controlled substances and is not considered addictive in a traditional way. It does not influence a brain as, for example, opioid drugs do. However, if you use the medication for a long time, you may need to increase the dosage in order to feel the same effect from the drug as you’ve experienced before taking lower dosages.
Because the drug does not interact with the opioid receptors (it targets the GABA neurotransmitters instead), it is not considered to be a very addictive drug. At the same time, however, it can produce withdrawal symptoms in people who abuse it. Also, it’s a sedative and can produce a high, so it’s entirely possible for individuals to become psychologically dependent on it.
Is Gabapentin a controlled substance?
Gabapentin is not classified as a controlled substance by FDA. However, you need your doctor’s prescription to buy Gabapentin online or a local pharmacy.
Where to buy Gabapentin generic online without prescription?
You can’t buy Gabapentin without prescription in your local pharmacy. However, you can easily order Gabapentin generic online with online prescription and distributed by online pharmacy. You can buy Gabapentin 300mg, 600mg or other dosages offered online.

Was prescribed for increasing anxiety, mood and alcohol withdrawal. Success! As a bonus helps with hot flashes. I am also on Lexapro. Gabapentin has helped me find a more normal calm and peace and no panic attacks.
My anxiety caused me to drink, and I have detoxed and with this med and therapy no longer have cravings to drink. I do have a dry mouth, but so what?!
The middle day dose is hard to remember, had to buy a pill box and set reminder on phone, no big deal. I feel normal! I am so grateful.
I was prescribed Gabapentin for a pinched sciatic nerve. I take 300 mg 3x a day. I went from laying flat on my back and almost screaming if I had to move to walking around within several days of starting the drug.
I have been taking it for about 2 months and have just started injections into my spine. I feel that the Gabapentin gave me my life back.
The only side effect that I noticed was the sleepiness. But after taking it for a week or so, this side effect lessened. I have gained about 5 pounds but I contribute this to lack of exercise. I would recommend this drug to anyone with a pinched sciatic nerve.
How do I pay and what steps do I have to take to make that payment happen???
Hey, Anthony,
You need pay money order to postman who deliver the package to you.
Been a cop for 25 years. Also worked for private contractors in Afghanistan. Needless to say, I retired with two gifts. PTSD and Alcohol abuse. Up to 1.75 (a handle as we drunks call it) a day.
Gabapentin came to the rescue (800 mg twice a day.) at first there was dizziness and I had to walk with a cane. On day 3, I normalized.
No cravings. Shakes disappeared. Nausea gone. Went to AA but that made me have cravings again after hearing the stories so, luckily, I stopped going. Miracle drug?
It was for me. Did not stop the PTSD though, but found that medical THC did the trick at night before bed. Sober for a month now. A first
I was prescribed Gabapentin for acute alcohol withdrawal, benzodiazepine withdrawal and anxiety. Currently taking 300mg x3 a day it has been working great, the withdrawals were bearable and now I’m finding the anxiety which once crippled me is completely gone. I can finally live in peace.”
Do not take gabapentin at the same time as antacids such as Maalox or Gaviscon. Separate administration by at least two hours. Take exactly as directed by your doctor, do not increase or decrease the dose without his or her advice.
Steve I was needing the order of gabapentin. I would like to ask you can next day shipping I will pay extra at usps
Hey, Brian
We can not do next day shipping because the doctors and the pharmacists need time review your health conditions
Best